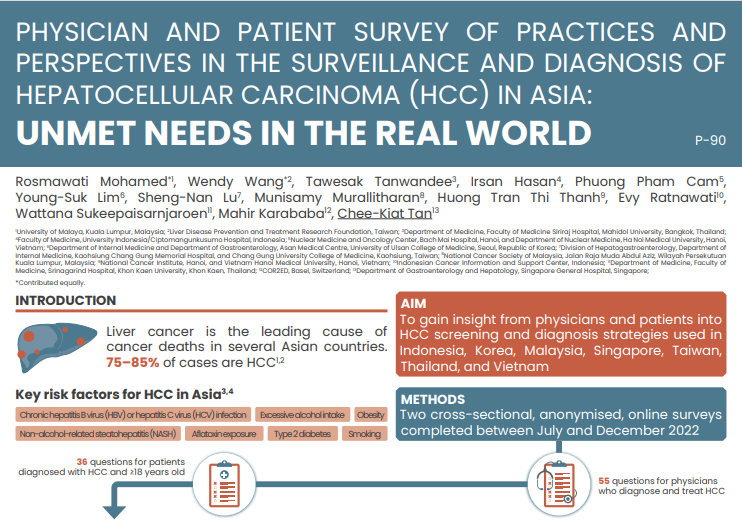
PARP Inhibitor Monotherapy
Well, hi everybody. I'm Neal Shore. I'm the Medical Director of Carolina Urologic Research Center and Chief Medical Officer at GenesisCare in the U.S.
Today we're going to talk about the role of PARP inhibitors monotherapy for mCRPC, specifically for patients who have homologous recombination repair HRR mutations.
The patient we're talking about today had a first line treatment with the doublet of androgen deprivation therapy and abiraterone, of course, there are multiple doublet options and even in some places, we have triplet options.
This particular patient had a quite advanced disease, high volume mHSPC. No known family history of cancer. It's important to get a good family history these days because we know that a significant family history, breast cancer, prostate cancer, pancreatic cancer, colorectal cancer, ovarian cancer, increases the risk of homologous repair alterations.
There are a lot of different potential options for patients who are mCRPC after they've gone undergone doublet therapy, this particular patient, ADT and abiraterone acetate and prednisone. A lot of different options.
It's very important to understand that we want to optimise the novel mechanisms of action and really not go forward with sequencing novel hormonal agents. We have data now that clearly demonstrates, whether it's the PROfound trial or CARD, that sequencing one NHA after another is nowhere near as effective as going to a novel MOA, such as a PARP inhibitor or a taxane, cabazitaxel in the CARD trial, PROfound demonstrating the value proposition for olaparib.
So if you have a patient who has an homologous recombinant repair or HRR mutation, they certainly could benefit from a PARP inhibitor. Testing is very important. Germline testing will pick up patients who have alterations early on. But if you have a negative germline, very important to move to somatic testing because otherwise, you'd miss 50% of your patients with metastatic disease. We can test with blood or with saliva for germline. Somatic - you can check with tissue-based testing, but if the tissue is not available, blood-based testing is very helpful.
We've recognised that genetic testing, particularly if you find a BRCA mutation, that these patients have clearly a worse prognosis. Patients who are BRCA positive, as this particular patient is, is now suitable for a PARP inhibitor monotherapy in first line or second line mCRPC.
We now have two PARP inhibitors that have been demonstrated level one evidence for their clinical utility for patients with castration-resistant disease, and this includes olaparib and rucaparib. And this has been demonstrated in the PROfound, TRITON2 and TRITON3 trials.
What we see is that with other trials as well, the use of sequencing is really not the best move.
PROfound demonstrated overall survival benefit, rPFS benefit, when we sequenced patients who had BRCA or ATM alterations as opposed to sequencing them with an androgen receptor targeted agent.
Similarly, a phase two study of TRITON2 and phase three trials of the TRITON3 demonstrated that offering a patient who had a BRCA alteration specifically as opposed to receiving, going against docetaxel but rather patients going on to receive a PARP inhibitor did markedly better.
There are unique adverse events of interest, inclusive of anaemia. CBCs need to be monitored. There are some mild to moderate GI side effects, but these are very manageable. So we have real clear indications now based upon EMA and FDA review. I think this is excellent.
And when we look at this particular case, this patient who was BRCA2, who then progressed after a doublet therapy of ADT and abiraterone, can now be an outstanding candidate for therapy in mCRPC with a PARP inhibitor.
So, I hope you found this particularly helpful. Thank you very much for your attention.











 19 MIN
19 MIN
 Jul 2024
Jul 2024  Downloadable
Downloadable